Experiment I
Part A Data Table
Color: Violet
| % Transmission |
Stopping Potential |
Approximate Charging Time |
| 100% |
1.89 V |
18.04 s |
| 80% |
1.85 V |
20.02 s |
| 60% |
1.81 V |
41.76 s |
| 40% |
1.80 V |
50.99 s |
| 20% |
1.79 V |
108.05 s |
Color: Green
| % Transmission |
Stopping Potential |
Approximate Charging Time |
| 100% |
0.97 V |
11.20 s |
| 80% |
1.02 V |
12.52 s |
| 60% |
0.97 V |
14.70 s |
| 40% |
0.97 V |
19.56 s |
| 20% |
0.97 V |
44.61 s |
Part B Data Table
| Light Color |
Stopping Potential |
| Yellow |
0.83 V |
| Green |
0.97 V |
| Blue |
1.56 V |
| Violet |
1.78 V |
| Ultraviolet |
2.06 V |
Experiment I Analysis Questions
1. When the % transmission was altered, the stopping potential did not vary all that much. In the violet color trial, we witnessed a negligible drop in the stopping potential as the % transmission dropped, but the overwhelming trend was that that the stopping potential remained more or less constant while the time to charge increased steadily.
2. Higher energy colors of light (i.e. those with a higher frequency, shorter wavelength) had a higher stopping potential than colors with lower frequencies.
3. This experiment supports a quantum model of light because with a wave theory of light, one would expect that with a more energetic wave (one with more amplitude and more brightness as a result), there would be more kinetic energy of the emitted electrons and thus the charging time would remain the same (since the same number of electrons would be emitted). But that wasn't what was observed. Instead, one finds that increasing transmission increases charging time because more electrons are emitted with more intensity of light. The only way this can be accounted for is by particle interactions between atoms in the metal and the incident photons, lending the photoelectric effect to be a prime example of the quantum model of light. There exists a slight drop in the measured stopping potential as the light intensity is decreased because some of the charge leaks off and the since the rate of charging decreases with a lower intensity, the equilibrium between charging and discharging is set a little bit lower.
Experiment II
Data Table
| First Order Color |
Wavelength (nm) |
Frequency (x1014 Hz) |
Stopping Potential (V) |
| Yellow |
577 |
5.2 |
0.83 |
| Green |
545 |
5.5 |
0.97 |
| Blue |
435 |
6.9 |
1.56 |
| Violet |
411 |
7.3 |
1.78 |
| Ultraviolet |
366 |
8.2 |
2.06 |
Sample Calculations:

Stopping Potential vs. Frequency
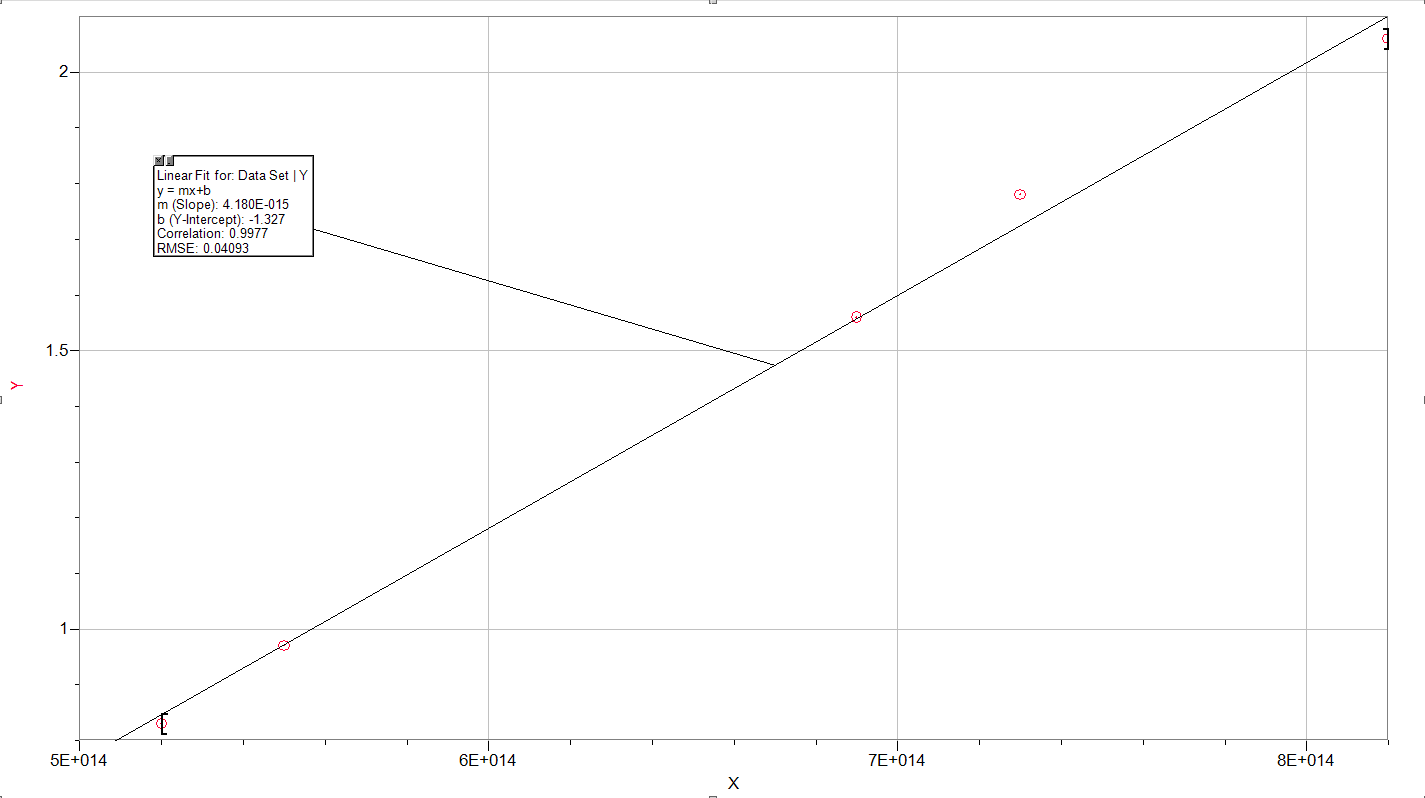



The work function,  , describes the minimum amount of energy required in order to produce an electron when impacted with an incident photon. This value changes depending on the type of metal for which the photoelectric effect is being observed, though. Planck's constant, h, is the quantum method for relating frequency of a photon to its energy through this proportionality constant.
, describes the minimum amount of energy required in order to produce an electron when impacted with an incident photon. This value changes depending on the type of metal for which the photoelectric effect is being observed, though. Planck's constant, h, is the quantum method for relating frequency of a photon to its energy through this proportionality constant.
Discussion Questions
1. No, one would expect the stopping voltage to be determined by the amplitude of the wave since the energy of the wave is dependent on its amplitude.
2. Yes, one would expect the stopping voltage to be determined by the intensity of the light since intensity is a function of the amplitude of the light and the total energy of the wave which would control the stopping voltage.
3. Yes, the incident wave must give off some of its energy to the electron (the electron soaking up the energy) so in theory, if the light was extremely weak, there would be a noticeable delay in the time when the incident light hits and the electrons begin to flow.
4. Yes, the total energy of the light particles are dependent on the photon's frequency by E=hf and the stopping voltage is dependent on the total energy of the particle's energy.
5. No, all that the intensity would do would alter the time it takes to reach the stopping potential but the total stopping voltage is a function of the energy of the incident particles, not a function of how many particles are incident to the surface.
6. No, the collision would be instantaneous (or almost entirely instantaneous) so the transfer of energy would also be instantaneous and the emission of photoelectrons would begin as soon as incident light hit the metal.
7. This experiment indubitably supports the quantum theory of light.
 icons at the top right corner of the subsection.
icons at the top right corner of the subsection.